What Did The Gold Foil Experiment Demonstrate
Rutherford performed gold foil experiment to understand that how negative and positive particles could Co exist in an atom. This was called the.

Evolution Of Atomic Theory Chemistry For Majors
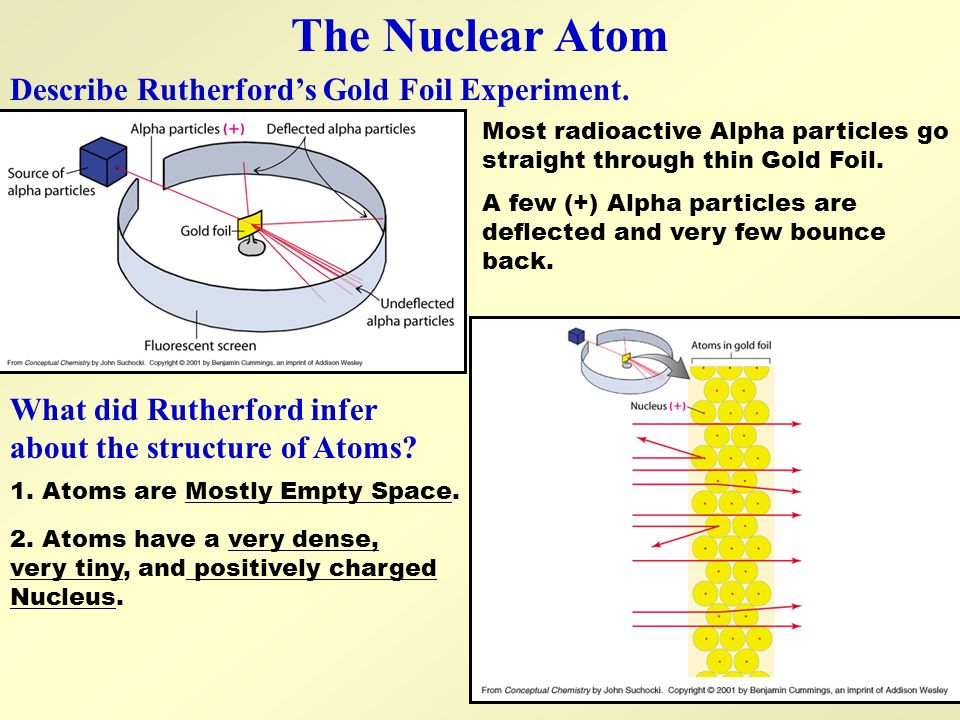
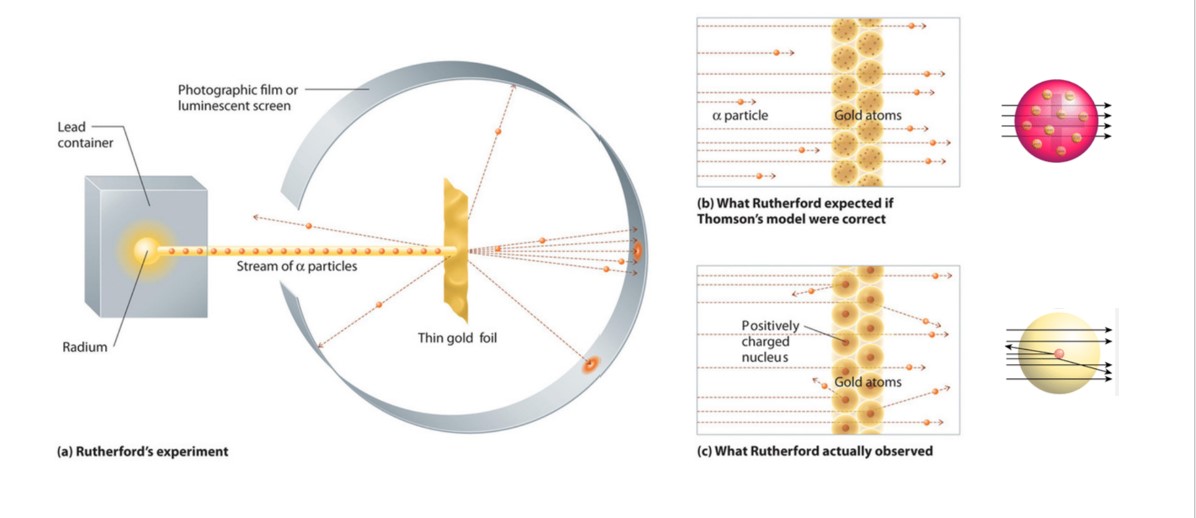
The particles were radiated through a thin slit to focus them so that they fired as a narrow beam at the gold foil behind which was a movable fluorescent screen to detect any particles that made it through.
What did the gold foil experiment demonstrate. Prior to his gold foil experiment scientists imagined the atom as a large area of positive charge with negative charges stuck on the outside. The Atomic model proposed by Ernest Rutherford was the Planetary Model and was devised on the basis of the Gold Foil Experiment. 1 See answer Advertisement Advertisement camilymjolesvr is waiting for your help.
Why did Dalton believe that atoms were indestructible and unchangeable. Rutherfords Gold Foil Experiment was an important experiment which revealed a lot about the structure of an atom and changed the worlds perspective of an Atomic Model. Most of the atom is empty space but the nucleus however small dictates the mass of the atom.
So what was Rutherfords Experiment and what did it reveal of the structure of an atom. He bombarded alpha particles on a 000004 cm thick gold foil. The density of mass in the nucleus.
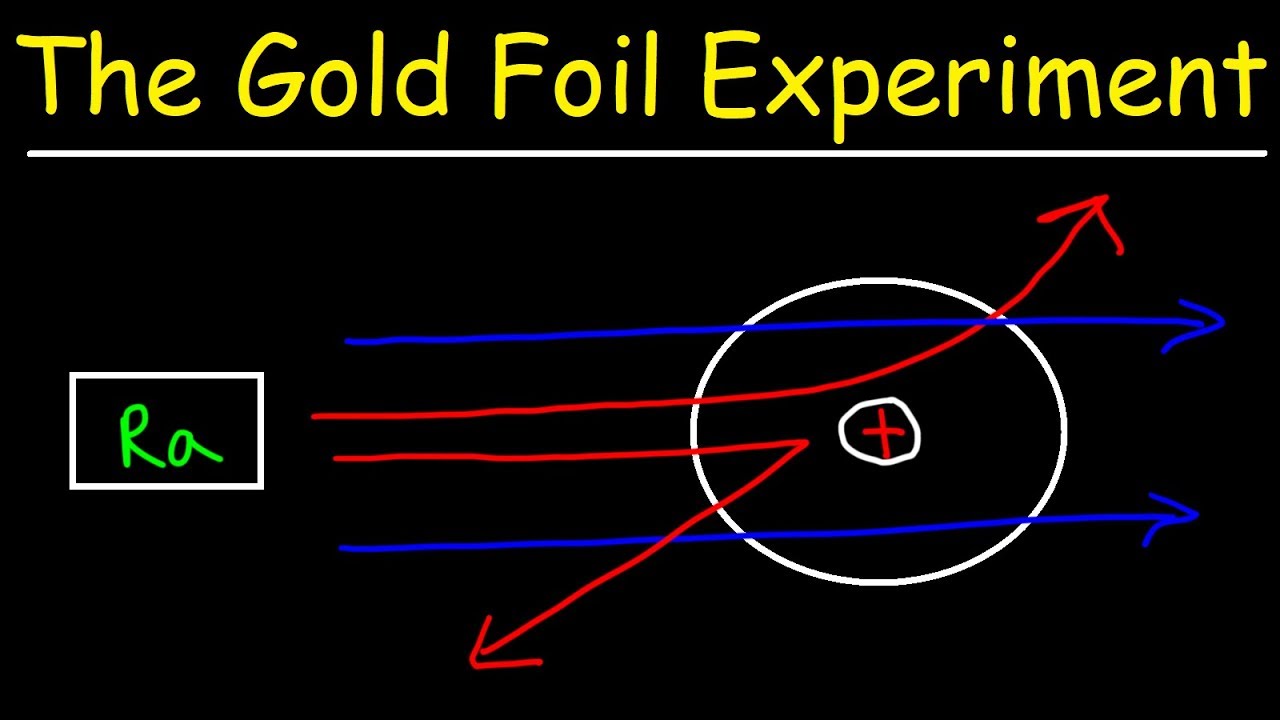
The alpha particles that were fired at the gold foil were positively charged. He beamed a ray of alpha particles onto a gold foil and. His two primary observations were.
Rutherfords conclusion came while observing particles 4 2He being fired in his famous gold foil experiment. Answer This experiment changed peoples way of thinking. It has also shown that the centre of the atom must be positively.
Add your answer and earn points. What was the first person to use the term atom. It has shown that mass of an atom is concentrated in the nucleus.
Where is most of the atoms mass located. What did the gold foil experiment demonstrate. What did Ernest Rutherford s gold foil experiment demonstrate about atoms.
Most of the atom is empty space but the nucleus however small dictates the mass of the atom. The gold foil experiment by Ernest Rutherford proved that the positive charge of an atom is located in the nucleus the small region that contributes most to the mass of the atom. He proposed a planetary model of the atom and concluded following results and demonstrated that.
Most of the time the alpha particles would pass through the foil without any change in their trajectories which is what was expected if JJ Thomsons plum pudding. The gold foil experiment by Ernest Rutherford proved that the positive charge of an atom is located in the nucleus the small region that contributes most to the mass of the atom. This chemistry video tutorial provides a basic introduction into Rutherfords Gold Foil Experiment.
Rutherfords gold foil experiments and other metal foil experiments involved firing positively charged alpha particles at a piece of goldmetal foil.
Unit 2 Atomic Structure Atomic Theory What Is A Theory Ppt Download

Rutherford S Atomic Model Chemistry For Non Majors

What Did Ernest Rutherford S Gold Foil Experiment Prove Brainly In

Rutherford S Gold Foil Experiment Video Khan Academy

Evolution Of Atomic Theory Chemistry For Majors
How Did Sir Ernest Rutherford Produce A Gold Foil That Was Approximately 1 000 Atoms Thick Quora
Rutherford S Gold Foil Experiment Quick And Simple Youtube

Rutherford Place Inside The Atom And What Experiment Did He Do To Prove Was The First To What Socratic

The Rutherford Geiger Marsden Experiment Physicsopenlab
Experimental Evidence For The Structure Of The Atom
Experimental Evidence For The Structure Of The Atom
How Did Rutherford Know That The Nucleus Was Positively Charged Socratic

Ernest Rutherford S Gold Foil Experiment Atomic Theory Modern Physics Chemistry Lessons

Rutherford S Gold Foil Experiment That Proved That Atoms Are Mainly Made Of Empty Space Rutherford Electro Atomic Theory Ernest Rutherford Chemistry Projects
Why Is The Rutherford S Of An Atom Called The Gold Foil Experiment Quora

Alpha Particle Scattering Experiment Ck 12 Foundation

Rutherford Gold Foil Experiment My Inter Academy Youtube

Rutherford Ernest Gold Foil Experiment Students Britannica Kids Homework Help
3 4 Rutherford S Experiment The Nuclear Model Of The Atom Chemistry Libretexts
Post a Comment for "What Did The Gold Foil Experiment Demonstrate"