How Did The Results Of Rutherford's Gold Foil Experiment Differ From His Expectations
Were to find deflected alpha particle rays at small angles. At the time when Rutherfords gold foil experiment was performed Thomsons plum pudding model was believed to be true at least by Rutherford himself and his students.

Rutherford Atomic Model Experiment Observations Limitations Video Lesson Transcript Study Com
Understand why the results of Rutherfords experiment rejected the plum pudding hypothesis of atomic structure.
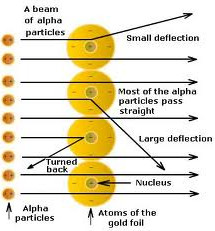
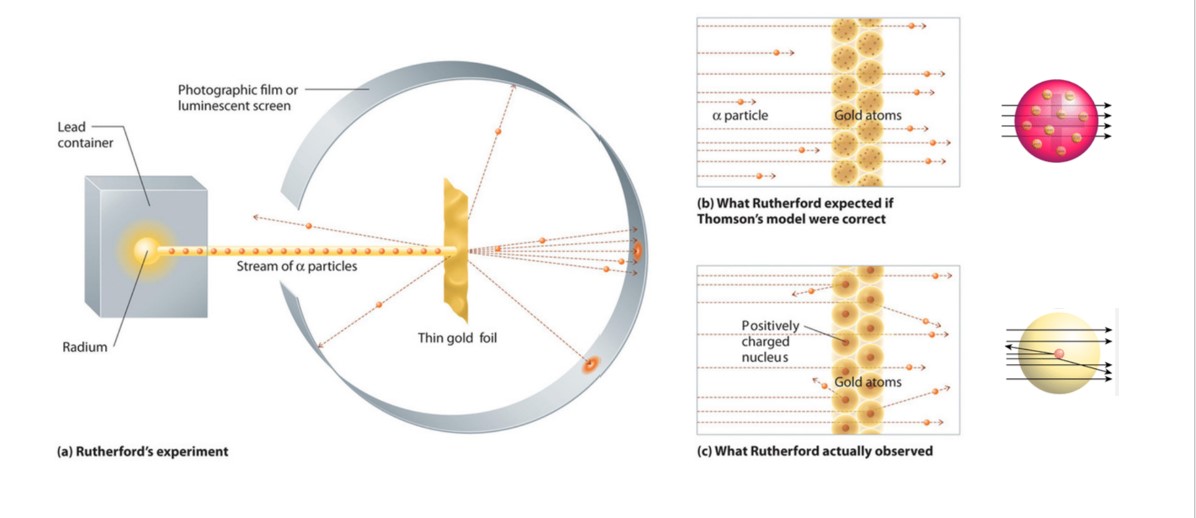
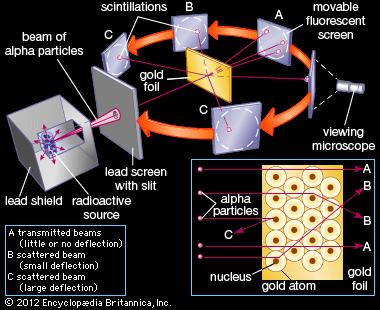
How did the results of rutherford's gold foil experiment differ from his expectations. In this model a vastly empty atom holds a tiny nucleus at the center surrounded by a cloud of electrons. Opposite the gold foil is a screen that emits a flash of light when struck by a particle. Thomson model argues that an atom is a sphere of positive charge with the negatively-charged electrons scattered like raisins in a pudding Rutherford and his students fully expected that an particle will pass through the gold foil with just a slight deflection on the angles since the particle is weakly positively charged.
Rutherford proposed that the atom is mostly empty space. Jun 08 2020 Rutherfords Gold FoilRutherfords Gold Foil Experiment proved the existance of a small massive center to atoms which would later be known as the nucleus of an atom. The GeigerMarsden experiments were performed between 1908 and 1913 by Hans Geiger of Geiger counter fame and Ernest Marsden a 20-year-old student who had not yet.
In 1909 two researchers in Ernest Rutherfords laboratory at the University of Manchester Hans Geiger and Ernest Marsden fired a beam of alpha particles at a thin metal foil. How did the results of Rutherfords gold-foil experiment differ from his expectations. In fact his experiment actually ushered in the atomic age.
Since the previous atomic model the Thomson model argues that an atom is a sphere of positive charge with the negatively-charged electrons scattered like raisins in a pudding Rutherford and his students fully expected that an alpha particle will pass through the gold foil with just a slight deflection on the angles since the alpha particle is weakly positively charged. Rutherfords Gold Foil Experiment was an important experiment which revealed a lot about the structure of an atom and changed the worlds perspective of an Atomic Model. His model explained why most of the particles passed straight through the foil.
Why did Rutherford use a thin gold foil. How did the actual results of Rutherfords gold foil experiment differ from the results he expected. So what was Rutherfords Experiment and what.
How did the results of rutherfords gold foil experiment. In Rutherford gold foil experiment positively charged alpha particles are bombarded on a thin gold foil. Of course ancient Greeks were also the first to.
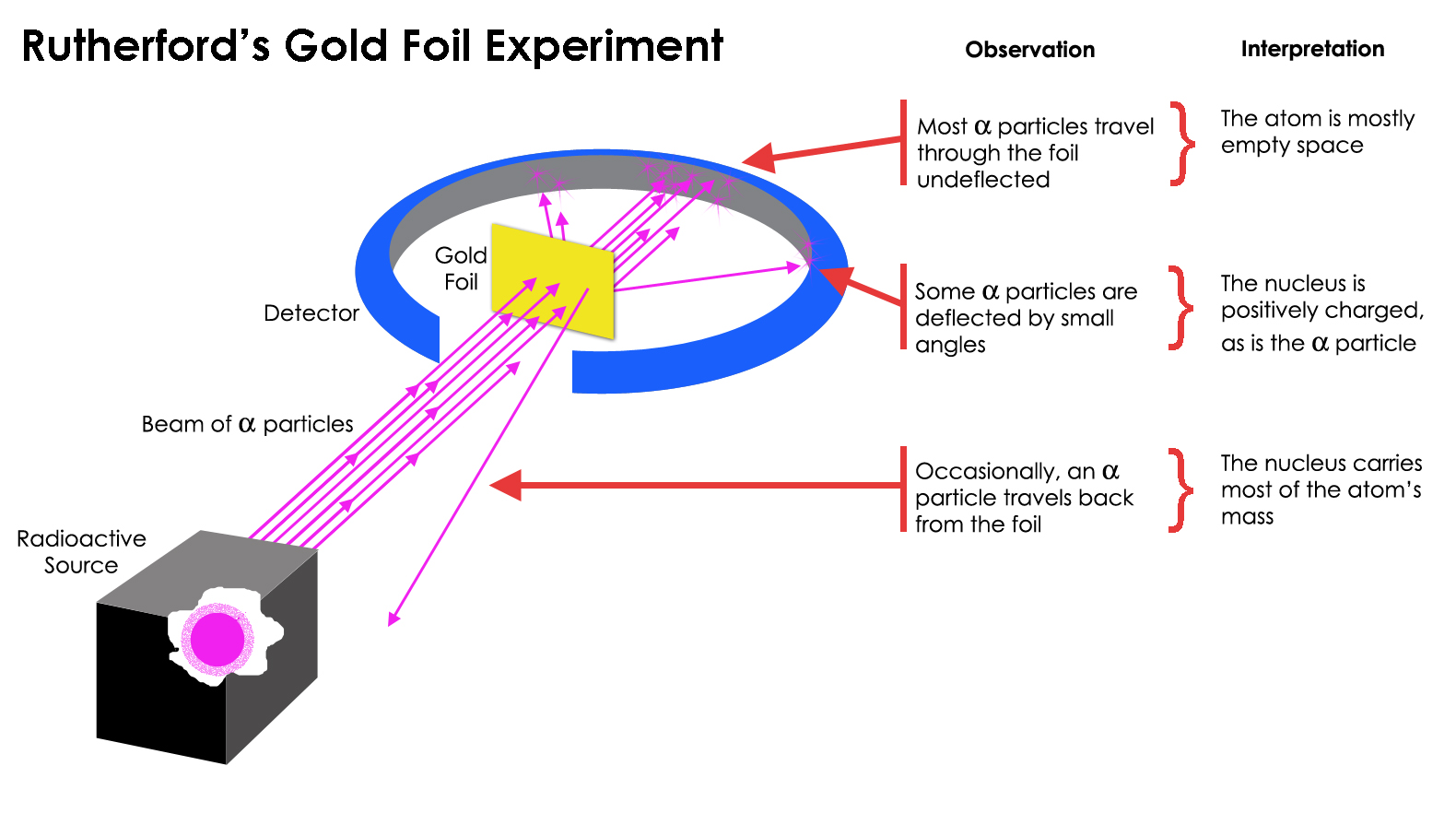
Rutherfords new model for the atom is based on the experimental results which were obtained from Geiger-Marsden experiments also called the Rutherford gold foil experiment. Were to find deflected alpha particle rays at small angles. The electrons revolve in circular orbits about a massive positive charge at the centre.
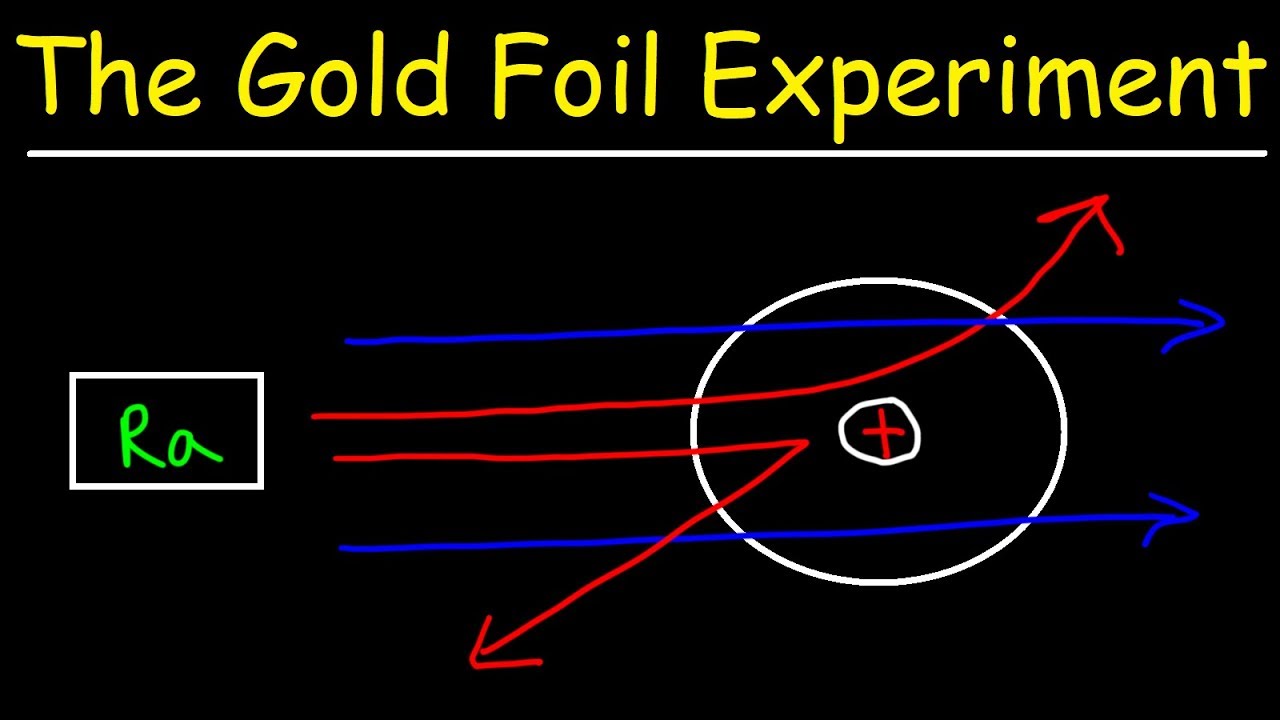
With this model in mind Rutherford predicted that most of the alpha particles will be deflected by at most a fraction of a degree sourced by this Wikipedia page but why. He used -particles because they are very energetic and are heavier than other particles. The small positive nucleus would deflect the few particles.
Ernest Rutherford Hans Geiger and Ernest Marsden carried out their Gold Foil Experiment to observe the effect of alpha particles on matter. The Atomic model proposed by Ernest Rutherford was the Planetary Model and was devised on the basis of the Gold Foil Experiment. Answer - Expected results.
The expected result of the experiment is that all of the alpha particles would pass through the gold foil minimum deflection. He further went on to reject the plum pudding model and developed a new atomic structure called the planetary model. In fact the ancient Greeks had predicted the existence of atoms around 500 BC.
Rutherford used gold for his scattering experiment because gold is the most malleable metal and he wanted the. They are approximately 4 times heavier than Hydrogen atoms. Gold Foil was bombarded with positively charged -particles.
How did the actual results of Rutherfords gold foil experiment differ from the results he expected. Rutherfords experiment and atomic model - David Darling. In this experiment Rutherford used Gold Foil which was extremely thin sheet not more than 1000 atoms thick.
The passing of many of the particles through suggested the condensed nucleus version of the atom model. They expected most of the alpha particles to pass easily through the foil with only a slight deflection. Better understand how the Rutherford gold foil experiment was done.
Gold Foil Experiment How did the actual results of Rutherfords gold foil ex. Understand why the results of this experiment led to the hypothesis that atoms have a small dense positive nucleus surrounded by clouds of negative electrons. His experiment produced amazing results and not just gold foil angel halos.
Answer - Expected results. As a result of his gold foil experiment Rutherfords atomic theory holds good even today. Principle of Rutherfords experiment By bombarding a very thin gold foil with alpha particles Hans Geiger and Ernest Marsden both students of Rutherford observed that a small fraction 1 in 8000 of these particles were deflected at large angle as if it bounced off a heavy obstacle.
In Rutherfords time the idea of the existence of atoms was not a new idea. Rutherfords diffraction experiment tests diffraction via a thin foil made of gold metal.
Rutherford Model Of An Atom Class 9 Structure Of An Atom

Rutherford S Gold Foil Experiment Chemistrygod

Pleasegive Mebrief Description About Atomic Model Presented Byrutherford Science Structure Of The Atom 8752085 Meritnation Com

Rutherford S Atomic Model Chemistry For Non Majors
3 4 Rutherford S Experiment The Nuclear Model Of The Atom Chemistry Libretexts

Rutherford S Gold Foil Experiment Chemistrygod
What Is The Source Of Alpha Particles In Rutherford Scattering Experiment
What Is Rutherford S Gold Foil Experiment
Rutherford S Gold Foil Experiment Quick And Simple Youtube
What Did Rutherford Expect To Happen In The Gold Foil Experiment Quora

Rutherford S Gold Foil Experiment Chemistrygod

Rutherford Atomic Theory Rutherford Experiment Atomic Theory Physics

Timeline Of The Evolution Of The Atomic Theory Preceden Atomic Theory Modern Physics Chemistry Lessons

Rutherford Place Inside The Atom And What Experiment Did He Do To Prove Was The First To What Socratic

Ernest Rutherford S Gold Foil Experiment Physics Lab Video Lesson Transcript Study Com
What Were Rutherford S Primary Observations In His Gold Foil Experiment And How Did He Explain Them Describe How He Modeled The Atom Socratic

Post a Comment for "How Did The Results Of Rutherford's Gold Foil Experiment Differ From His Expectations"